Medical Devices Regulations: Manage Inspection Dates with Inventory Software
On this page you will find information on the following topics:
- Keeping an Eye on Medical Devices Regulations and Maintenance With Inventory Software
- Medical Devices Regulations – How Often Does Your Inventory Need to Be Inspected?
- Medical Devices Regulations – What Does It Involve?
- Who Is Allowed to Carry Out Medical Devices Regulations?
- Medical Device Inspection: X-Ray Equipment and Other Highly Sensitive Devices

Medical devices regulations always kept in view thanks to smart inventory software
Keeping an Eye on Medical Devices Regulations and Maintenance With Inventory Software
There are hardly any more sensitive areas of work than health care. Despite all economic constraints, it is still primarily not about profits and losses, but about people’s health and lives. The inventory plays a special role in this. It must be ready for use in an emergency. Regular maintenance and adherence to safety checks are a basic prerequisite for this.
The working time of nursing staff is extremely valuable. Do not burden your highly qualified nursing staff with unnecessary bureaucratic procedures. Inventory management must be simple, fast and intuitive. Timly is an all-in-one solution for inventory management. The software shows its particular strengths in the combination of all integrated functions. These not only allow a permanent overview of the locations and condition of the inventory.
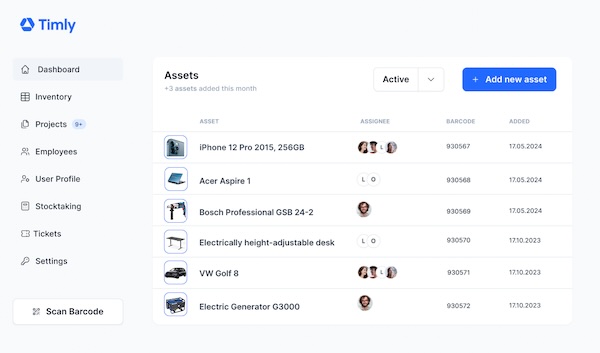
It also shows necessary maintenance and required qualifications of the assigned personnel. Inventory software like Timly works on a simple principle. Every work equipment and other asset, from office chairs to vehicles, is recorded when it is put into operation. It is given a QR code, with the help of which the profile of the item can subsequently be called up by simply scanning it. All the necessary information is stored in it.
In the case of furniture, this may only be the name and location. For complex medical equipment, on the other hand, descriptions, operating instructions, maintenance dates and test certificates can be stored. The profiles are deliberately kept so flexible that they can be adapted for every conceivable object. The data is stored in a cloud memory. The provider takes care of its GDPR-compliant administration.
Your employees can access it with any internet-enabled device. Timly offers an optimised app for smartphones and tablets. When taking over a device and preparing medical treatments, your employees are thus able to check the proper condition of each device used – for example, compliance with all maintenance and inspection dates. Your staff can report problems immediately using the app. This can be combined with a ticketing system that optimises the repair process.
Medical Devices Regulations – How Often Does Your Inventory Need to Be Inspected?
The intervals of each inspection must be calculated in such a way that defects that occur according to experience can be detected in good time. According to medical devices regulations, an inspection must be carried out after 24 months at the latest.
The wording of the ordinance puts the onus on the operator. It is up to him to determine inspection intervals for each specific case. These can be highly individual. Inventory software with an integrated maintenance planner provides your employees with an instrument that allows them to determine at any time whether a medical device inspection is due. The risk of a safety check being lost in the stressful daily routine of medical facilities is minimised.
Asset Management Software in Use by Our Customers
The Timly software is continuously evolving to meet the needs of our customers. In various success stories, we show you how Timly optimizes processes in companies, thereby saving significant effort. With Timly, inventory management becomes child’s play.

Optimized Device Management With Innovative Self-Inventory
SodaStream is the world market leader for water sparkling systems for domestic use and has a lot of IT equipment at its various locations. Many colleagues now work from their home offices. A digital solution for the efficient management of IT end devices became necessary...

Panasonic x Timly: Driving Technological Innovation
One of the most remarkable aspects of human ingenuity is our ability to innovate. Innovation is embedded in the DNA of consumer electronics giant Panasonic, which has diversified into a number of sectors, from heavy industry to construction...

Manage Video Equipment Efficiently Without Much Effort
The Hamburg media company always does outstanding journalistic work and is characterized by independent reporting. In order to maintain journalistic quality, the teams work with highly specialized devices – these need to be managed efficiently...

Smart City Asset Management – Timly in Use at DIGOOH
The core business of DIGOOH Media GmbH in Cologne is to manage digital city light posters (DCLP) for outdoor use in various cities in Germany. The challenge here lies in making the client’s communication message always available at the right time, in the right place...
(No credit card required)
Medical Devices Regulations – What Does It Involve?
Medical devices regulations must be carried out in accordance with the generally recognised rules of technology. Detailed protocols with responsible persons and possible measured values must be prepared.
This means that maintenance and inspection instructions must also be individually recorded. You can do this with Timly for each device category in the profile of the medical device. All authorised employees have uncomplicated access to the test documentation with their smartphones or tablets stored in the workspace.
Who Is Allowed to Carry Out Medical Devices Regulations?
Depending on the device, it is always explicitly specified which group of persons may carry out a medical device inspection. In general it can be said that the persons carrying out the test must have the relevant professional training and must not be subject to any professional instructions.
In some cases, special certificates are required. If you use your own employees for the inspection procedures, you can also store their suitability in the Timly software. It can also be used to record the validity of any training certificates that may be required.
Over 600 Companies, Schools and Cities Rely on Timly
Medical Device Inspection: X-Ray Equipment and Other Highly Sensitive Devices
In the manufacturing industry, breakdowns of machines and tools are annoying and may cause financial losses.
In the medical sector, on the other hand, there is a concrete risk that improperly working equipment will endanger the health of patients and staff.
Working in the X-ray sector, for example, is risky. Diagnoses and measures to be taken partly depend on the results of complex medical devices.

Keep track of all medical devices regulation dates with Timly
Those responsible in the medical sector should therefore do everything to ensure that medical inventory functions reliably and safely. Inventory software can effectively support you and your staff in this.
Similar Blog Posts:
Book an online demo - free and without obligation - or create your free trial account directly.