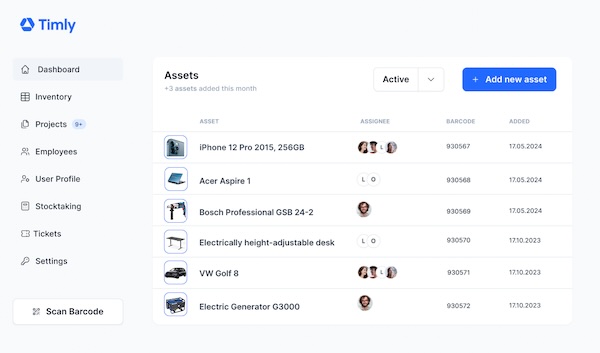
Medical Device Safety: Ensuring Compliance with Timly’s Asset Tracking Solution
In this article:
- Streamlining Medical Device Management with Inventory Software
- Enhancing Medical Device Compliance with Digital Documentation
- Medical Device Safety: Identifying Equipment Requiring Mandatory Testing
- Timly’s Solution: Safeguarding Delicate Medical Devices
- Maintaining Oversight of Devices and Personnel with Timly’s Trust Chain
- Navigating Complex Regulations in Medical Device Management
- Minimising Costs and Effort in Safety Testing
- Special Requirements in the Medical and Healthcare Industry

Ensuring medical device safety is made easy with Timly’s inventory management software and maintenance planner.
Streamlining Medical Device Management with Inventory Software
Medical device safety and compliance is of paramount importance for patient safety. Not surprisingly, EU regulations mandate strict classification and conformity assessment of medical devices to guarantee their proper functioning. Adhering to these stringent regulations necessitates meticulous organization. This includes following diverse inspection schedules and fulfilling specific criteria set for inspection personnel.
However, for healthcare professionals, the reliability of medical devices in urgent situations transcends regulatory compliance. In high-stakes scenarios, patient safety and well-being take precedence. Timly’s software is instrumental in supporting both staff and administrators in this aspect. Its inventory management software adeptly documents all medical inventory, ensuring vital information is instantly accessible on-site. Furthermore, the automated maintenance management system offered by Timly streamlines the scheduling of maintenance tasks. This efficiency allows healthcare workers to dedicate more time to their fundamental responsibilities, underlining the pivotal role of medical device safety and compliance in healthcare.
Enhancing Medical Device Compliance with Digital Documentation
To keep track of these critical deadlines, Timly equips each item in its inventory with a dedicated calendar. This feature allows for the scheduling of maintenance and inspection dates, with automated reminders ensuring timely compliance. These reminders can be directed to responsible personnel or even to external service providers if needed.
Inspections and maintenance of medical devices should only be conducted by personnel who are adequately trained and equipped with the necessary technical expertise. Timly’s comprehensive approach to managing both personnel and equipment shines here. The app enables the storage of essential qualification certificates for employees, as well as certificates and documentation for managed items, including digital copies of relevant regulatory compliance documents. This system allows for the easy input and retrieval of information related to inspection personnel in Timly’s digital personnel records.
Test protocols and evidence of inspections are systematically stored in the profile of each medical device within the app. Authorized staff can access these records at any time, ensuring transparency and safety in the management of sensitive medical equipment.
Over 600 Companies, Schools and Cities Rely on Timly
Medical Device Safety: Identifying Equipment Requiring Mandatory Testing
Additionally, equipment such as ventilators, syringe pumps, MRI scanners, and invasive patient monitors must undergo regular inspections. Timly’s recording and management system is particularly beneficial in the healthcare sector. Its flexibility allows for custom categorization to meet specific requirements, facilitating comprehensive management of a diverse range of medical equipment, each with its unique parameters. This adaptability is crucial in upholding safety standards and ensuring regulatory compliance.
Timly’s Solution: Safeguarding Delicate Medical Devices
With Timly, medical operators can establish a digital chain of trust for the regulation of medical devices. This process is crucial due to the sensitive nature of these devices. Key steps in this chain include:
- Conducting functional tests during setup
- Providing comprehensive training to personnel based on safety guidelines and usage instructions,
- Ensuring all procedures are carried out by manufacturer-trained personnel
These steps are essential to maintain the functioning and reliability of medical devices. Timly’s unique blend of personnel and equipment management creates a robust framework for medical device management. Its holistic approach simplifies adherence to safety standards and regulatory requirements, thus making the management of these sensitive devices more straightforward and efficient.
Maintaining Oversight of Devices and Personnel with Timly’s Trust Chain
Timly’s chain-of-trust system is initiated the moment a medical device is registered in the system. Each registered item is marked with a QR code sticker, linking it to a digital file that contains all necessary information about the device, including usage instructions, checklists, and inspection records. This comprehensive database is securely stored in cloud-based storage.
The platform employs a robust user authorization system to ensure data security and relevance. Each employee has access only to the information pertinent to their role, aligning with GDPR compliance standards and eliminating the need for external network access. The versatility of mobile technology comes into play here; employees can use smartphones or tablets to scan the QR codes and access the device profiles instantly.
This seamless integration of device and employee skills management fosters complete transparency in the regulation of medical devices. Authorized personnel can easily verify the condition and compliance status of any equipment using their mobile devices before commencing their duties. They can also check if fellow staff members possess the necessary training, qualifications, or manufacturer-issued certifications, thereby ensuring every aspect of medical device safety and compliance is meticulously monitored.
Medical Device Management: Monitoring Equipment and Staff Credentials with Timly
Navigating Complex Regulations in Medical Device Management
Beyond the standard requirements set by EU Medical Devices Regulations (MDR) and subsequent revisions apply to medical devices with measurement functions. These regulations aim to ensure that such devices adhere to permissible error limits in their measurements.
This represents a specialized obligation for certain types of medical equipment, necessitating thorough oversight of measurement accuracy. To address this, Timly’s maintenance calendar is designed to manage varying inspection schedules efficiently, such as those for safety-related and metrological checks.
The implementation of these additional, specific regulations for devices with measuring functions mirrors the complexity often encountered in medical device management. Timly’s software simplifies this complexity by digitizing the management of diverse regulatory deadlines, providing a streamlined solution for ensuring compliance across multiple regulatory requirements.

Minimising Costs and Effort in Safety Testing
Ensuring a clear history of conducted safety inspections is crucial for protecting both patients and medical staff. Eliminating cumbersome administrative tasks not only saves time and focus for employees but also makes processes more efficient and cost-effective. For instance, creating an inventory list is effortlessly manageable with Timly. All necessary fields, such as device information and individual identification numbers, can be flexibly configured.
Moreover, Timly simplifies the process of maintaining accurate records of device locations as required. Any changes, such as loans to different departments, can be updated promptly by responsible parties. In practice, facilities like the Clinique DELC in Biel, Switzerland, have successfully implemented Timly. Administrators particularly appreciate the benefits it brings to quality management, attributing this to the intelligent capturing and linking of information.
Special Requirements in the Medical and Healthcare Industry
The medical and healthcare sector faces unique challenges unlike any other industry. Therefore, it’s wise for administrators to implement established, software-based support systems. Such systems not only relieve the burden on nursing staff but also save costs while enhancing safety for both patients and employees. The availability of crucial information where it’s needed most significantly reduces the risk of errors.
Technical tools like automated maintenance planners and maintenance management software for managing regular inspections can alleviate some of the heavy responsibilities shouldered by the already overburdened staff. With Timly, safety inspections become just one of many routine tasks, streamlining processes and ensuring compliance without adding extra strain on personnel.
Similar Blog Posts:
Book an online demo - free and without obligation - or create your free trial account directly.